Abstract
Background: Primary graft failure (GF) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation (HSCT). Second allogeneic stem cell transplantation (HSCT2) is one of the potential treatments.
Methods: We assessed the outcome of adult patients (pts) with acute leukemia (AL) in remission (CR1- CR2) that failed to engraft (ANC<0.5 x 109/L) > day 28 after the first transplant (HSCT1) and received a second transplant (HSCT2) while in the same disease status (from 28 days to 120 days after HSCT1). HSCT2 was performed between 2000 and 2021.
Results: In total, 243 pts were included: 182 (74.9%) with AML and 61(25.1%) with ALL, respectively. The median age was 44.8 (range, 18.4-75.2) years. Cytogenetic risk in AML (MRC classification) was favorable/ intermediate, and adverse in 92 (50.5%) and 30 (16.5%) pts, respectively (missing data-60 pts, 33.0%). 71.6% of pts were in CR1 while 28.4% were in CR2. Donors of HSCT1 were matched siblings (MSD) in 9.1%, unrelated (UD) in 39.9%, other family members (Haplo) in 35%, and cord blood (CB) in 16% of pts. HSCT1 grafts were bone marrow in 25.1%, peripheral blood in 58.4%, combined in 0.4% and single or double CB in 9.5%, and 16.6% of transplants. At HSCT1, conditioning was myeloablative (MAC) in 58.4% and reduced intensity (RIC) in 41.6%. GVHD prophylaxis was calcineurin inhibitor (CNI)-based in 70.9% and was post-transplant cyclophosphamide (PTCy) in combination with CNI in 29.1%. 46.1% and 14.8% of the HSCTs were with in vivo and ex vivo T-cell depletion, respectively. The time from first to second transplantation was 48 (range, 28-120) days. Donors at HSCT2 were the same as in HSCT1 in 49% while a different donor was used in 51% of the cases. They were MSD in 10.7%, UD in 31.7%, Haplo in 45.7%, and CB in 11.9% of pts. Conditioning was RIC in 80.4% and MAC in 19.6% of HSCT2. PTCy was used in 27.7% and in vivo and ex vivo T-cell depletion was performed in 49.1% and 13.5% of HSCT2, respectively.
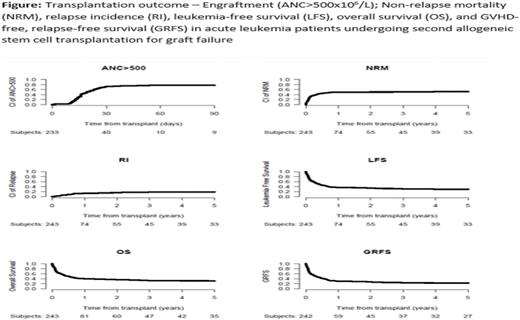
Engraftment post HSCT2 was achieved by 73.7% of the pts, 78.6% in pts with AML vs. 59% in pts with ALL with day 60 ANC > 0.5 x 109/L in 77.1% of pts; 82.1% in pts with AML vs. 62.1% in those with ALL, p=0.034. Day 180 incidence of acute (a) GVHD II-IV and III-IV was 23.2% and 8.2%, respectively. The 5-year total and extensive chronic (c) GVHD was 22.3%, and 10.1%, respectively. Both acute and cGVHD did not differ between pts with AML vs. those with ALL. The 5-year NRM was 51.6%. Infections were the main cause of death in 47% of pts who died, followed by GF and GVHD at 7.9% each, with no difference between AML and ALL. The 5 -year RI was 18.8%. The 5 -year LFS, OS and GRFS were 29.6%, 30.7% and 22.4%, respectively (Figure). In multivariable analysis, being transplanted at CR2 vs. CR1, increased age (per 10-years), lower KPS (<90) at HSCT2 and receiving MAC at HSCT1 were adverse prognostic factors for NRM, LFS and OS. Hazard ratio (HRs) for NRM were 2.26 (95% CI 1.41-3.63, p=0.0007), HR=1.31 (95% CI 1.09-1.54, p=0.005), HR=2.17 (95% CI 1.30-3.70, p=0.003) and HR=2.63 (95% CI 1.52-4.55, p=0.0006), respectively. HRs for LFS were 2.21 (95% CI 1.43-3.33, p=0.0002), HR=1.2 (95% CI 1.03-1.41, p=0.02), HR=1.92 (95% CI 1.23-3.03, p=0.004) and HR=2.0 (95% CI 1.27-3.13, p=0.003), respectively. HRs for OS were 2.38 (95% CI 1.57-3.6, p<0.0001), HR=1.26 (95% CI 1.07-1.48, p=0.005), HR=2.04 (95% CI 1.30-3.23, p=0.002) and HR=2.38 (95% CI 1.47-3.85, p=0.0003), respectively. Being transplanted at CR2 vs CR1, lower KPS (<90) and receiving MAC at HSCT1 were adverse prognostic factors for GRFS with HR of 1.82 (95% CI 1.23-2.71, p=0.003), HR=1.49 (95% CI 1-2.22, p=0.047), and HR=1.59 (95% CI 1.02-2.5, p= 0.039), respectively. In AML patients, adverse cytogenetics was an adverse prognostic factor for RI, LFS, OS, GRFS, and aGVHD II-IV with a HR of 8.22 (95% CI 3.01-22.46, p<0.0001), HR=2.48 (95% CI 1.43-4.31, p=0.001), HR=2.06 (95% CI 1.16-3.65, p= 0.014), HR=2.34 (95% CI 1.38-3.97, p=0.002) and HR=3.32 (95% CI 1.36-8.13, p=0.008), respectively.
Conclusions: HSCT2 can rescue about a third of AL pts with GF. However, transplant-related mortality is very high with a 5-year mortality of about 50%. The outcomes of HSCT2 for GF in AML and ALL are similar. Being transplanted at CR2 vs. CR1, lower KPS, MAC at HSCT1, increased age and adverse cytogenetics (for AML) are adverse prognostic factors. A different donor at HSCT2 seems not to improve results. GF remains a grave complication of transplantation that awaits novel ideas and strategies.
Disclosures
Labopin:Jazz Pharmaceuticals: Honoraria. Kuball:Novartis: Research Funding; Miltenyi Biotech: Patents & Royalties: novel CAR T and engineering isolation strategies, Research Funding; GADETA: Current equity holder in private company, Patents & Royalties: on gdTCR engineering strategies and targets , Research Funding. Leleu:Takeda: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; BMS: Honoraria; Janssen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Platzbecker:Takeda: Honoraria; Silence Therapeutics: Honoraria; Janssen: Honoraria; Jazz: Honoraria; BMS/Celgene: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Geron: Honoraria. Ciceri:Kite Pharma: Consultancy. Mohty:Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal